

新疆农业科学 ›› 2024, Vol. 61 ›› Issue (9): 2277-2284.DOI: 10.6048/j.issn.1001-4330.2024.09.023
• 植物保护·微生物·畜牧兽医·土壤肥料 • 上一篇 下一篇
安哲1,2( ), 牛瑞昌2, 朱香镇2, 王丽2, 张开心2, 李东阳2, 姬继超2, 牛林2, 高雪珂2, 雒珺瑜2, 崔金杰2(
), 牛瑞昌2, 朱香镇2, 王丽2, 张开心2, 李东阳2, 姬继超2, 牛林2, 高雪珂2, 雒珺瑜2, 崔金杰2( ), 马德英1(
), 马德英1( )
)
收稿日期:2024-02-20
出版日期:2024-09-20
发布日期:2024-10-09
通信作者:
马德英(1968-),女,新疆乌鲁木齐人,教授,博士,硕士生/博士生导师,研究方向为有害生物绿色防控,(E-mail)mdynd@163.com;作者简介:安哲(1996-),女,内蒙古乌兰察布人,硕士研究生,研究方向为农业昆虫与害虫防治,(E-mail)anzhe1206@126.com
基金资助:
AN Zhe1,2( ), NIU Ruichang2, ZHU Xiangzhen2, WANG Li2, ZHANG Kaixin2, LI Dongyang2, JI Jichao2, NIU Lin2, GAO Xueke2, LUO Junyu2, CUI Jinjie2(
), NIU Ruichang2, ZHU Xiangzhen2, WANG Li2, ZHANG Kaixin2, LI Dongyang2, JI Jichao2, NIU Lin2, GAO Xueke2, LUO Junyu2, CUI Jinjie2( ), MA Deying1(
), MA Deying1( )
)
Received:2024-02-20
Published:2024-09-20
Online:2024-10-09
Supported by:摘要:
【目的】研究不同菌型棉蚜体内微生物种类与丰度的差异。【方法】通过HiSeq平台对不同菌型棉田棉蚜体内共生菌的16S rRNA基因V3~V4区进行高通量测序,分析绿盲蝽体内共生菌的种类与多样性。【结果】对照组与沙雷氏菌型、汉密尔顿菌型棉蚜的优势菌门均为变形菌门,相对物种丰度分别占97.42%、95.55%和92.78%。对照组与试验组的优势菌科均为肠杆菌科,但相对丰度有所差异,相对丰度分别占96.14%、81.285%和84.22%。汉密尔顿菌型棉蚜与沙雷氏菌型棉蚜其体内汉密尔顿菌属与沙雷氏菌属微生物丰度与对照组相比显著升高,分别占比77.40%和12.04%。【结论】棉田中含有沙雷氏菌与汉密尔顿菌的棉蚜其体内微生物丰度受到显著影响,其体内汉密尔顿菌属与沙雷氏菌属相对丰度显著上升。
中图分类号:
安哲, 牛瑞昌, 朱香镇, 王丽, 张开心, 李东阳, 姬继超, 牛林, 高雪珂, 雒珺瑜, 崔金杰, 马德英. 棉田不同菌型棉蚜体内微生物多样性分析[J]. 新疆农业科学, 2024, 61(9): 2277-2284.
AN Zhe, NIU Ruichang, ZHU Xiangzhen, WANG Li, ZHANG Kaixin, LI Dongyang, JI Jichao, NIU Lin, GAO Xueke, LUO Junyu, CUI Jinjie, MA Deying. Analyze the microbial diversity of cotton aphids with different bacterial types in cotton fields[J]. Xinjiang Agricultural Sciences, 2024, 61(9): 2277-2284.
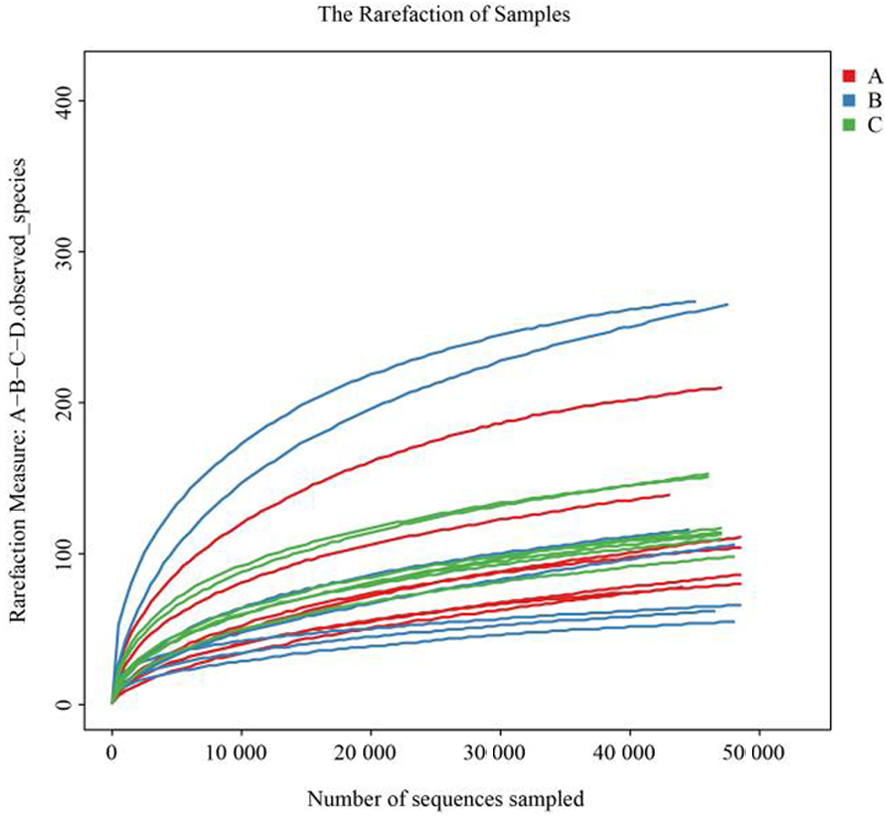
图1 Obs指数稀释曲线 注:A:对照组棉蚜种群;B:沙雷氏菌棉蚜种群;C:汉密尔顿菌棉蚜种群,下同
Fig.1 Obs exponent dilution curve Note:A:Control group Aphis gossypii populations;B:Serratia Aphis gossypii populations;C:Hamiltonella Aphis gossypii populations,the same as below
| Samples | Number of reads | Number of OTUs | Chao | ACE | Shannon | Simpson | Coverage |
|---|---|---|---|---|---|---|---|
| A | 377 680 | 59 | 163.925 40 | 205.339 59 | 0.588 4 | 0.777 78 | 0.999 12 |
| B | 395 108 | 56 | 177.230 86 | 209.957 57 | 1.047 65 | 0.644 03 | 0.999 15 |
| C | 428 742 | 14 | 175.649 41 | 192.584 21 | 0.928 44 | 0.622 17 | 0.999 1 |
表1 各样本中的序列数(条)和Alpha多样性指数
Tab.1 Sequence number and Alpha diversity index for each sample
| Samples | Number of reads | Number of OTUs | Chao | ACE | Shannon | Simpson | Coverage |
|---|---|---|---|---|---|---|---|
| A | 377 680 | 59 | 163.925 40 | 205.339 59 | 0.588 4 | 0.777 78 | 0.999 12 |
| B | 395 108 | 56 | 177.230 86 | 209.957 57 | 1.047 65 | 0.644 03 | 0.999 15 |
| C | 428 742 | 14 | 175.649 41 | 192.584 21 | 0.928 44 | 0.622 17 | 0.999 1 |
| [1] | Crotti E, Balloi A, Hamdi C, et al. Microbial symbionts: a resource for the management of insect-related problems[J]. Microbial Biotechnology, 2012, 5(3): 307-317. |
| [2] | Engl T, Kaltenpoth M. Influence of microbial symbionts on insect pheromones[J]. Natural Product Reports, 2018, 35(5): 386-397. |
| [3] | Baumann P. Biology bacteriocyte-associated endosymbionts of plant sap-sucking insects[J]. Annual Review of Microbiology, 2005, (59): 155-189. |
| [4] | Gündüz E A, Douglas A E. Symbiotic bacteria enable insect to use a nutritionally inadequate diet[J]. ProceedingsBiological Sciences, 2009, 276(1658): 987-991. |
| [5] | Von Dohlen C D, Moran N A. Molecular data support a rapid radiation of aphids in the Cretaceous and multiple origins of host alternation[J]. Biological Journal of the Linnean Society, 2000, 71(4): 689-717. |
| [6] | Nakabachi A, Ishikawa H. Provision of riboflavin to the host aphid, Acyrthosiphonpisum, by endosymbiotic bacteria, Buchnera[J]. Journal of Insect Physiology, 1999, 45(1): 1-6. |
| [7] | Fukatsu T, Hosokawa T. Capsule-transmitted gut symbiotic bacterium of the Japanese common plataspid stinkbug, Megacoptapunctatissima[J]. Applied and Environmental Microbiology, 2002, 68(1): 389-396. |
| [8] | Chen D Q, Purcell A H. Occurrence and transmission of facultative endosymbionts in aphids[J]. Current Microbiology, 1997, 34(4): 220-225. |
| [9] | Tsuchida T, Koga R, Fujiwara A, et al. Phenotypic effect of “CandidatusRickettsiellaviridis,” a facultative symbiont of the pea aphid (Acyrthosiphonpisum), and its interaction with a coexisting symbiont[J]. Applied and Environmental Microbiology, 2014, 80(2): 525-533. |
| [10] | Heyworth E R, Ferrari J. A facultative endosymbiont in aphids can provide diverse ecological benefits[J]. Journal of Evolutionary Biology, 2015, 28(10): 1753-1760. |
| [11] | West S A, Cook J M, Werren J H, et al. Wolbachia in two insect host-parasitoid communities[J]. Molecular Ecology, 1998, 7(11): 1457-1465. |
| [12] | Fukatsu T, Tsuchida T, Nikoh N, et al. Spiroplasma symbiont of the pea aphid, Acyrthosiphonpisum (Insecta: Homoptera)[J]. Applied and Environmental Microbiology, 2001, 67(3): 1284-1291. |
| [13] | Jousselin E, Cœurd’Acier A, Vanlerberghe-Masutti F, et al. Evolution and diversity of Arsenophonus endosymbionts in aphids[J]. Molecular Ecology, 2013, 22(1): 260-270. |
| [14] | Chen D Q, Campbell B C, Purcell A H. A new rickettsia from a herbivorous insect, the pea aphid Acyrthosiphonpisum (Harris)[J]. Current Microbiology, 1996, 33(2): 123-128. |
| [15] | N., A., Moran, et al. Evolutionary Relationships of Three New Species of Enterobacteriaceae Living as Symbionts of Aphids and Other Insects[J]. Applied and Environmental Microbiology, 2005, 71(6): 3302-3310. |
| [16] | Montllor C B, Maxmen A, Purcell A H. Facultative bacterial endosymbionts benefit pea aphids Acyrthosiphonpisum under heat stress[J]. Ecological Entomology, 2002, 27(2): 189-195. |
| [17] | Slosser J E, Pinchak W E, Rummel D. A review of known and potential factors affecting the population dynamics of the cotton aphid[J]. Southwestern Entomologist, 1989. |
| [18] | Jacobson R J, Croft P. Strategies for the control of Aphis gossypii glover (Hom.: Aphididae) with Aphidiuscolemaniviereck (Hym.: Braconidae) in protected cucumbers[J]. Biocontrol Science and Technology, 1998, 8(3): 377-387. |
| [19] | Hullé M, Chaubet B, Turpeau E, et al. Encyclop’Aphid: a website on aphids and their natural enemies[J]. EntomologiaGeneralis, 2020, 40(1): 97-101. |
| [20] | Balabanidou V, Grigoraki L, Vontas J. Insect cuticle: a critical determinant of insecticide resistance[J]. Current Opinion in Insect Science, 2018, (27): 68-74. |
| [21] | Broderick N A, Raffa K F, Handelsman J. Midgut bacteria required for Bacillus thuringiensis insecticidal activity[J]. Proceedings of the National Academy of Sciences of the United States of America, 2006, 103(41): 15196-15199. |
| [22] | Kikuchi Y, Hayatsu M, Hosokawa T, et al. Symbiont-mediated insecticide resistance[J]. Proceedings of the National Academy of Sciences of the United States of America, 2012, 109(22): 8618-8622. |
| [23] | Liu X D, Guo H F. Importance of endosymbionts Wolbachia and Rickettsia in insect resistance development[J]. Current Opinion in Insect Science, 2019, (33): 84-90. |
| [24] | Ghanim M, Kontsedalov S. Susceptibility to insecticides in the Q biotype of Bemisiatabaci is correlated with bacterial symbiont densities[J]. Pest Management Science, 2009, 65(9): 939-942. |
| [25] | Kontsedalov S, Zchori-Fein E, Chiel E, et al. The presence of Rickettsia is associated with increased susceptibility of Bemisiatabaci (Homoptera: Aleyrodidae) to insecticides[J]. Pest Management Science, 2008, 64(8): 789-792. |
| [26] | Zhang S, Su H H, Jiang W L, et al. Symbiotic microbial studies in diverse populations of Aphis gossypii, existing on altered host plants in different localities during different times[J]. Ecology and Evolution, 2021, 11(20): 13948-13960. |
| [27] | Oliver K M, Degnan P H, Burke G R, et al. Facultative symbionts in aphids and the horizontal transfer of ecologically important traits[J]. Annual Review of Entomology, 2010, (55): 247-266. |
| [28] | Meseguer A S, Manzano-Marín A, Coeur d’Acier A, et al. Buchnera has changed flatmate but the repeated replacement of co-obligate symbionts is not associated with the ecological expansions of their aphid hosts[J]. Molecular Ecology, 2017, 26(8): 2363-2378. |
| [29] | Oliver K M, Russell J A, Moran N A, et al. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps[J]. Proceedings of the National Academy of Sciences of the United States of America, 2003, 100(4): 1803-1807. |
| [30] | Russell J A, Moran N A. Costs and benefits of symbiont infection in aphids: variation among symbionts and across temperatures[J]. ProceedingsBiological Sciences, 2006, 273(1586): 603-610. |
| [31] | Mago T, Salzberg S L. FLASH: fast length adjustment of short reads to improve genome assemblies[J]. Bioinformatics, 2011, 27(21): 2957-2963. |
| [32] | Edgar R C. UPARSE: highly accurate OTU sequences from microbial amplicon reads[J]. Nature Methods, 2013, 10(10): 996-998. |
| [33] | Edgar R C, Haas B J, Clemente J C, et al. UCHIME improves sensitivity and speed of chimera detection[J]. Bioinformatics, 2011, 27(16): 2194-2200. |
| [34] | Caporaso J G, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data[J]. Nature Methods, 2010, (7): 335-336. |
| [35] | Edgar R C. Search and clustering orders of magnitude faster than BLAST[J]. Bioinformatics, 2010, 26(19): 2460-2461. |
| [36] | Schloss P D, Westcott S L, Ryabin T, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities[J]. Applied and Environmental Microbiology, 2009, 75(23): 7537-7541. |
| [37] | Wilkinson T J, Huws S A, Edwards J E, et al. CowPI: a rumen microbiome focussed version of the PICRUSt functional inference software[J]. Frontiers in Microbiology, 2018, (9): 1095. |
| [38] | Chaves S, Neto M, Tenreiro R. Insect-symbiont systems: from complex relationships to biotechnological applications[J]. Biotechnology Journal, 2009, 4(12): 1753-1765. |
| [39] | Schloss P D, Delalibera I Jr, Handelsman J, et al. Bacteria associated with the guts of two wood-boring beetles: Anoplophoraglabripennis and Saperdavestita (Cerambycidae)[J]. Environmental Entomology, 2006, 35(3): 625-629. |
| [40] | Behar A, Yuval B, Jurkevitch E. Gut bacterial communities in the Mediterranean fruit fly (Ceratitis capitata) and their impact on host longevity[J]. Journal of Insect Physiology, 2008, 54(9): 1377-1383. |
| [41] | Zhang S, Luo J Y, Wang L, et al. Bacterial communities in natural versus pesticide-treated Aphis gossypii populations in North China[J]. MicrobiologyOpen, 2019, 8(3): 652-657. |
| [42] | Xu T T, Chen J, Jiang L Y, et al. Diversity of bacteria associated with Hormaphidinae aphids (Hemiptera: Aphididae)[J]. Insect Science, 2021, 28(1): 165-179. |
| [43] | Arneodo J D, Ortego J. Exploring the bacterial microbiota associated with native South American species ofAphis(Hemiptera: Aphididae)[J]. Environmental Entomology, 2014, 43(3): 589-594. |
| [44] | Gauthier J P, Outreman Y, Mieuzet L, et al. Bacterial communities associated with host-adapted populations of pea aphids revealed by deep sequencing of 16S ribosomal DNA[J]. PLoS One, 2015, 10(3): e0120664. |
| [45] | Tsuchida T, Koga R, Shibao H, et al. Diversity and geographic distribution of secondary endosymbiotic bacteria in natural populations of the pea aphid, Acyrthosiphonpisum[J]. Molecular Ecology, 2002, 11(10): 2123-2135. |
| [46] | Hansen A K, Moran N A. Aphid genome expression reveals host-symbiont cooperation in the production of amino acids[J]. Proceedings of the National Academy of Sciences of the United States of America, 2011, 108(7): 2849-2854. |
| [47] | Koga R, Tsuchida T, Fukatsu T. Changing partners in an obligate symbiosis: a facultative endosymbiont can compensate for loss of the essential endosymbiont Buchnera in an aphid[J]. ProceedingsBiological Sciences, 2003, 270(1533): 2543-2550. |
| [48] | Oliver K M, Higashi C H. Variations on a protective theme: Hamiltonelladefensa infections in aphids variably impact parasitoid success[J]. Current Opinion in Insect Science, 2019, (32): 1-7. |
| [49] | Li Q, Sun J X, Qin Y G, et al. Reduced insecticide susceptibility of the wheat aphid Sitobion miscanthi after infection by the secondary bacterial symbiont Hamiltonelladefensa[J]. Pest Management Science, 2021, 77(4): 1936-1944. |
| [50] | Li Q, Fan J, Sun J X, et al. Anti-plant defense response strategies mediated by the secondary symbiont Hamiltonelladefensa in the wheat aphid Sitobion miscanthi[J]. Frontiers in Microbiology, 2019, (10): 2419. |
| [1] | 张庭军, 李字辉, 崔豫疆, 孙孝贵, 陈芳. 微生物菌剂对棉花生长及土壤理化性质的影响[J]. 新疆农业科学, 2024, 61(9): 2269-2276. |
| [2] | 陈芳, 李字辉, 孙孝贵, 张庭军. 不同剂量的微生物菌剂对加工番茄产量及品质的影响[J]. 新疆农业科学, 2024, 61(9): 2285-2289. |
| [3] | 帕孜丽耶·艾合麦提, 王新勇, 周燕, 宋彬, 玉苏甫·阿不力提甫. 微生物菌剂对核桃叶片生理及光合特性的影响[J]. 新疆农业科学, 2024, 61(9): 2299-2306. |
| [4] | 乔雅洁, 付慧鑫, 乔雪, 孟新涛, 张婷, 潘俨. 不同贮藏温度条件下鲜牛肉品质的变化规律[J]. 新疆农业科学, 2024, 61(9): 2323-2329. |
| [5] | 陈芳, 李字辉, 王兵跃, 孙孝贵, 张庭军. 微生物菌剂对冬小麦生长发育及产量的影响[J]. 新疆农业科学, 2024, 61(8): 1853-1860. |
| [6] | 刘会芳, 韩宏伟, 庄红梅, 王强, 高阿香, 王浩. 增施微生物肥对设施黄瓜土壤微生物多样性的影响[J]. 新疆农业科学, 2024, 61(7): 1727-1737. |
| [7] | 贾东海, 宋贤明, 顾元国, 李强, 曾幼玲, 苗昊翠, 郭美丽, 侯献飞. 化肥减量配施微生物菌肥对膜下滴灌红花生长发育及产量的影响[J]. 新疆农业科学, 2024, 61(4): 781-790. |
| [8] | 王贺亚, 罗静静, 王康, 王瑞楠, 王旭, 高光瑞, 房艳. 不同微生物菌剂对甜菜根腐病的防效及产量影响[J]. 新疆农业科学, 2024, 61(2): 448-454. |
| [9] | 王伟, 张仁福, 刘海洋, 丁瑞丰, 梁革梅, 姚举. 不同抗性遗传背景棉蚜氟啶虫胺腈及啶虫脒抗性品系转录组分析[J]. 新疆农业科学, 2024, 61(12): 3078-3088. |
| [10] | 于吕健, 阳瑾, 丁宇, 李晓曼, 刘峰娟, 范盈盈, 梁红玉, 焦子伟, 王成. 石榴果实表面真菌的分离鉴定及致病性测定[J]. 新疆农业科学, 2024, 61(1): 148-155. |
| [11] | 罗林毅, 陈瑞杰, 阮向阳, 任晓辉, 曲奥, 苏海婷, 冶军. 微生物菌剂对滴灌棉田土壤养分和棉花产量及品质的影响[J]. 新疆农业科学, 2024, 61(1): 26-33. |
| [12] | 王丹丹, 李燕, 张庆银, 李世东, 庞永超, 马琨芝, 马龙, 牛瑞生, 钟增明, 齐连芬, 师建华. 不同微生物菌处理对番茄土壤微生物多样性的影响[J]. 新疆农业科学, 2023, 60(9): 2248-2257. |
| [13] | 徐静, 石书兵, 秦小钢, 朱军. 核桃林间作西红花对其土壤微生物数量的动态变化[J]. 新疆农业科学, 2023, 60(8): 2022-2027. |
| [14] | 薛正轩, 蔡志平, 张智健, 彭天祥, 黄志伟, 黄恩泽, 王佩玲, 陆宴辉. 基于铷元素标记技术分析多异瓢虫在甘草带与棉田间的迁移范围[J]. 新疆农业科学, 2023, 60(7): 1741-1747. |
| [15] | 岳丽, 王卉, 山其米克, 再吐尼古丽·库尔班, 涂振东. 基于高通量测序的甜高粱青贮饲料中微生物群落分析[J]. 新疆农业科学, 2023, 60(11): 2742-2750. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||