

新疆农业科学 ›› 2022, Vol. 59 ›› Issue (6): 1418-1428.DOI: 10.6048/j.issn.1001-4330.2022.06.013
• 土壤肥料·园艺特产·贮藏保鲜加工·种质资源 • 上一篇 下一篇
王强1,2( ), 刘会芳1, 韩宏伟1, 庄红梅1, 王柏柯1, 王娟1, 杨涛1, 王浩1(
), 刘会芳1, 韩宏伟1, 庄红梅1, 王柏柯1, 王娟1, 杨涛1, 王浩1( ), 秦勇2(
), 秦勇2( )
)
收稿日期:2021-10-01
出版日期:2022-06-20
发布日期:2022-07-07
通信作者:
王浩(1970-),男,山东人,研究员,研究方向为设施蔬菜栽培与生理, (E- mail) wanghao183@163.com;作者简介:王强(1983-),男,甘肃人,副研究员,研究方向为蔬菜栽培生理与逆境胁迫,(E-mail) wangqiang201004@sina.com
基金资助:
WANG Qiang1,2( ), LIU Huifang1, HAN Hongwei1, ZHUANG Hongmei1, WANG Baike1, WANG Juan1, YANG Tao1, WANG Hao1(
), LIU Huifang1, HAN Hongwei1, ZHUANG Hongmei1, WANG Baike1, WANG Juan1, YANG Tao1, WANG Hao1( ), QIN Yong2(
), QIN Yong2( )
)
Received:2021-10-01
Published:2022-06-20
Online:2022-07-07
Supported by:摘要:
【目的】 盐胁迫是造成番茄产量损失和品质严重下降的关键非生物胁迫之一,研究番茄耐盐的分子机制,为认识番茄幼苗应答盐胁迫分子调控机制奠定基础。【方法】 研究利用番茄耐盐渐渗系IL-7-5-5和番茄盐敏感M82为试材,采用同位素相对标记与绝对定量(TMT)技术结合定量蛋白质组平行反应监测(PRM)技术,对200 mM盐胁迫12 h的番茄幼苗叶片进行蛋白质组学研究,筛选出盐胁迫响应显著的潜在靶标蛋白。【结果】 (1)共鉴定出286个差异显著表达蛋白(DEPs)。盐胁迫下IL-7-5-5鉴定到191个DEPs,其中119个表达上调,72个表达下调。在M82中鉴定出157个DEPs,其 中84个表达上调,73个表达下调。维恩图分析显示,有129和95个差异蛋白分别特异于IL-7-5-5和M82。有62个显著差异蛋白共表达,其中 28 个在IL-7-5-5和M82中均上调,15 个均为下调,表现出对盐胁迫的一致响应。19个显著差异蛋白在表达相反,有5个蛋白质在ST中下调,在SS中上调,14个差异蛋白在ST中上调,在SS中下调;(2)番茄盐反应蛋白种类诱导能力强,主要与代谢过程、单组织过程以及细胞过程有关,细胞组成主要涉及细胞、细胞器、分子复合物和膜,基因本体分子功能显示,这些差异性蛋白主要参与催化活性、绑定和分子功能调控。(3)选择11个显著差异蛋白PRM验证结果与TMT 定量表现出相同的趋势。差异蛋白包括 A0A3Q7E8T9、A0A3Q7EK65、A0A3Q7FY19、A0A3Q7G430、A0A3Q7ITH0、A0A3Q7J1Y7、P05116、Q43779、A0A3Q7F8W6、A0A3Q7GKU3、A0A3Q7J0Z4,可能是番茄幼苗耐盐的潜在靶标蛋白。【结论】 研究采用 TMT 结合 PRM 技术,筛选出番茄幼苗响应盐胁迫差异表达蛋白。
中图分类号:
王强, 刘会芳, 韩宏伟, 庄红梅, 王柏柯, 王娟, 杨涛, 王浩, 秦勇. 基于 TMT和PRM 技术筛选番茄响应盐胁迫差异表达蛋白[J]. 新疆农业科学, 2022, 59(6): 1418-1428.
WANG Qiang, LIU Huifang, HAN Hongwei, ZHUANG Hongmei, WANG Baike, WANG Juan, YANG Tao, WANG Hao, QIN Yong. Screening Differentially Expressed Proteins in Response to Salt Stress of Tomato Leaves Based on TMT and PRM Techniques[J]. Xinjiang Agricultural Sciences, 2022, 59(6): 1418-1428.
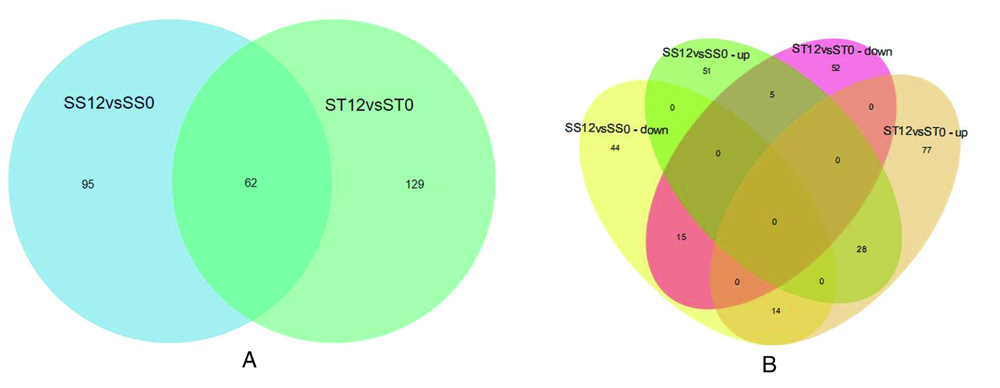
图1 2种番茄品种差异表达蛋白比较 注:(A)差异表达蛋白(DEPs)比较的数量。(B)两个番茄幼苗DEPs蛋白的维恩图
Fig.1 Comparative analysis of differentially expressed proteins in two tomato varieties. Note: (A) Number of up-regulated and down-regulated differentially expressed proteins (DEPs). (B) Venn diagram of tomato DEPs in two maize varieties.
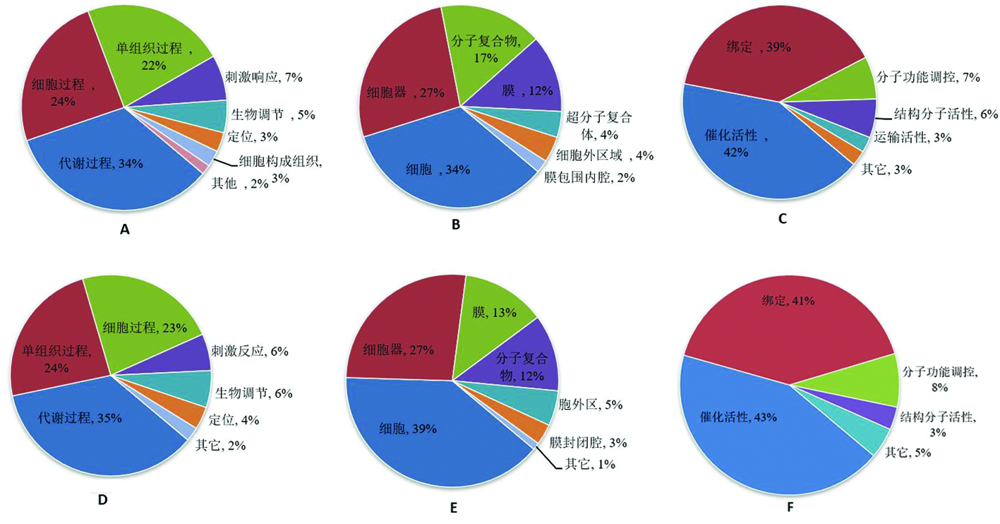
图2 差异蛋白的 GO 功能分类 注:A、D分别代表SS12 vs SS0、ST12 vs ST0生物学过程;图B、E分别代表SS12 vs SS0、ST12 vs ST0细胞成分;图C、F分别代表SS12 vs SS0、ST12 vs ST0分子功能
Fig.2 Gene ontology enrichment analysis of differentially expressed protein Note:A and D represent the biological processes of SS12 vs SS0 and ST12 vs ST0 respectively.Figure2- B and E represent SS12 vs SS0 and ST12 vs ST0 cell components respectively.Figure 2-C and F represent the molecular functions of SS12 vs SS0 and ST12 vs ST0 respectively
| 蛋白号 Protein Accession | 蛋白质的描述 Protein description | SS12/SS0 TMT PRM | ST12/ST0 TMT PRM | ||
|---|---|---|---|---|---|
| A0A3Q7E8T9 | 过氧化物酶* | / | / | 1.56 | 2.72 |
| A0A3Q7EK65 | 精氨酸酶 2* | / | / | 1.97 | 3.98 |
| A0A3Q7FY19 | 可能乙酰辅酶a乙酰转移酶* | / | / | 1.64 | 2.51 |
| A0A3Q7G430 | 黄氧素脱氢酶 | 0.64 | 0.54 | 3.37 | 3.25 |
| A0A3Q7ITH0 | 过氧化物酶 | 1.82 | 1.24 | 1.64 | 1.89 |
| A0A3Q7J1Y7 | 可能多胺氧化酶 2 | 0.81 | 0.61 | 2.26 | 6.47 |
| P05116 | 1-氨基环丙烷-1-羧酸氧化酶1 | 3.41 | 4.96 | 1.67 | 2.67 |
| Q43779 | 超氧化物歧化酶* | 1.53 | 1.8 | / | / |
| A0A3Q7F8W6 | 微管蛋白α链 | 0.6 | 0.48 | 1.57 | 1.63 |
| A0A3Q7GKU3 | 40S核糖体蛋白S13样* | / | / | 1.62 | 1.44 |
| A0A3Q7J0Z4 | 延长因子1α | 0.57 | 0.3 | 2.22 | 3.48 |
表1 PRM 和 TMT 的定量结果比较
Table 1 Comparison of PRM and TMT quantification result
| 蛋白号 Protein Accession | 蛋白质的描述 Protein description | SS12/SS0 TMT PRM | ST12/ST0 TMT PRM | ||
|---|---|---|---|---|---|
| A0A3Q7E8T9 | 过氧化物酶* | / | / | 1.56 | 2.72 |
| A0A3Q7EK65 | 精氨酸酶 2* | / | / | 1.97 | 3.98 |
| A0A3Q7FY19 | 可能乙酰辅酶a乙酰转移酶* | / | / | 1.64 | 2.51 |
| A0A3Q7G430 | 黄氧素脱氢酶 | 0.64 | 0.54 | 3.37 | 3.25 |
| A0A3Q7ITH0 | 过氧化物酶 | 1.82 | 1.24 | 1.64 | 1.89 |
| A0A3Q7J1Y7 | 可能多胺氧化酶 2 | 0.81 | 0.61 | 2.26 | 6.47 |
| P05116 | 1-氨基环丙烷-1-羧酸氧化酶1 | 3.41 | 4.96 | 1.67 | 2.67 |
| Q43779 | 超氧化物歧化酶* | 1.53 | 1.8 | / | / |
| A0A3Q7F8W6 | 微管蛋白α链 | 0.6 | 0.48 | 1.57 | 1.63 |
| A0A3Q7GKU3 | 40S核糖体蛋白S13样* | / | / | 1.62 | 1.44 |
| A0A3Q7J0Z4 | 延长因子1α | 0.57 | 0.3 | 2.22 | 3.48 |
| [1] |
Zhai Y, Yang Q, Hou M. The Effects of Saline Water Drip Irrigation on Tomato Yield, Quality, and Blossom-End Rot Incidence-A 3a Case Study in the South of China[J]. PLoS ONE, 2015, 10:e0142204.
DOI URL |
| [2] |
Munns R, Tester M. Mechanisms of salinity tolerance[J]. Annual Review of Plant Biology, 2008, 59(0):651-681.
DOI URL |
| [3] |
Pérez-Alfocea F, Balibrea M E, Cruz A.S, et al. Agronomical and physiological characterization of salinity tolerance in a commercial tomato hybrid[J]. Plant and Soil, 1996, 180(2):251-257.
DOI URL |
| [4] |
Zhu J K. Salt and drought stress signal transduction in plants[J]. Annual Review of Plant Biology, 2002, 53(1):247-273.
DOI URL |
| [5] |
Zhu J K. Abiotic stress signaling and responses in plants[J]. Cell, 2016, 167(2):313-324.
DOI URL |
| [6] |
Ishikawa T, Shabala S. Control of xylem Na(+) loading and transport to the shoot in rice and barley as a determinant of differential salinity stress tolerance[J]. Physiologia Plantarum, 2018, 165(3):619-631.
DOI URL |
| [7] |
Zhang Z, Mao C Y, Shi Z, et al. The amino acid metabolic and carbohydrate metabolic pathway play important roles during salt-stress response in tomato[J]. Frontiers in Plant Science, 2017, 8:1231.
DOI PMID |
| [8] |
Manaa A, Faurobert M, Valot B, et al. Effect of salinity and calcium on tomato fruit proteome[J]. Omics A Journal of Integrative Biology, 2013, 17(6):338-352.
DOI URL |
| [9] |
Keutgen A J, Pawelzik E. Contribution of amino acids to strawberry fruit quality and their relevance as stress indicators under NaCl salinity[J]. Food Chemistry, 2008, 111(3):642-647.
DOI URL |
| [10] |
Galli v, Messias R D S, Perin E C, et al. Mild salt stress improves strawberry fruit quality[J]. LWT-Food Science and Technology, 2016, 73:693-699.
DOI URL |
| [11] | Lu S W, Li T L, Jing J. Effects of tomato fruit under Na+-salt and Cl-salt stresses on sucrose metabolism[J]. African Journal of Agricultural Research, 2010, 5(16):2227-2231. |
| [12] |
Chen C, Plant A. Salt-induced protein synthesis in tomato roots: the role of ABA[J]. Journal of Experimental Botany, 1999, 50(334):677-687.
DOI URL |
| [13] |
Amini F, Ehsanpour A, Hoang Q, et al. Protein pattern changes in tomato under in vitro salt stress[J]. Russian Journal of Plant Physiology, 2007, 54(4):464-471.
DOI URL |
| [14] |
Chen S, Gollop N, Heuer B. Proteomic analysis of salt-stressed tomato (Solanum lycopersicum) seedlings: effect of genotype and exogenous application of glycinebetaine[J]. Journal of Experimental Botany, 2009, 60(7):2005-2019.
DOI URL |
| [15] |
Ibrahimova U F, Mammadov A C, Feyziyev Y M. The effect of NaCl on some physiological and biochemical parameters in Triticum aestivum L. genotypes[J]. Plant Physiol Report, 2019, 24(3):370-375.
DOI URL |
| [16] |
Pailles Y, Awlia M, Julkowska M M, et al. Diverse Traits Contribute to Salinity Tolerance of Wild Tomato Seedlings from the Galapagos Islands[J]. Plant Physiology, 2020, 182(1):534-546.
DOI URL |
| [17] |
Pang Q S, Chen S, Dai Y, et al. Comparative proteomics of salt tolerance in Arabidopsis thaliana and Thellungiella halophila[J]. Proteome Research, 2010, 9(5):2584-2599.
DOI URL |
| [18] |
Zhou J, Palmer J, Zhou S, et al. Differential root proteome expression tomato genotypes with contrasting drought tolerance exposed to dehydration[J]. Journal of the American Society for Horticultural Science, 2013, 138(2):131-141.
DOI URL |
| [19] |
Peterson A C, Russell J D, Bailey D J. Parallel reaction monitoring for high resolution and high mass accuracy quantitative, targeted proteomics[J]. Molecular & Cellular Proteomics: MCP, 2012, 11(11):1475-1488.
DOI URL |
| [20] |
Wang Z X, Shang P, Li Q G, et al. iTRAQ-based proteomic analysis reveals key proteins affecting muscle growth and lipid deposition in pigs[J]. Scientific reports, 2017, 7(1):46717.
DOI URL |
| [21] | Ira G W. The function of ribosomal protein outside the ribosome[J]. Life of Chemistry, 1997, 17(1):23-25. |
| [22] |
Tadepalli A, Roney O L. Ribosomal protein S25 mRNA partners with MTF-1 and La to provide a p53-mediated mechanism for survival or death[J]. The Journal of Biological Chemistry, 2002, 277(6):4147.
DOI URL |
| [23] | 孙伟, 李燕, 赵彦修, 等. 盐地碱蓬延伸因子(SsEF-1α)的克隆与表达分析[J]. 西北植物学报, 2004, 24(9):1657-1661. |
| SUN Wei, LI Yan, ZHAO Yanxiu, et al. Isolation and characterizing of a cDNA clone encoding an elongation factorEF-1α from halophyte Suaeda salsa[J]. Acta Botanica Boreali-Occidentalia Sinica, 2004, 24(9):1657-1661. | |
| [24] |
Padaria J C, Yadav R, Tarafdar A, et al. Molecular cloning and characterization of drought stress responsive abscisic acid-stress-ripening (Asr 1) gene from wild jujube, Ziziphus nummularia (Burm.f.) Wight & Arn[J]. Molecular biology reports, 2016, 43(8):849-859.
DOI URL |
| [25] |
Feng Z J, Xu Z S, Sun J, et al. Investigation of the ASR family in foxtail millet and the role of ASR1 in drought oxidative stress tolerance[J]. Plant Cell Reports, 2016, 35(1):115-128.
DOI URL |
| [26] |
Goldgur Y, Rom R, Ghirlando D, et al. Desiccation and zinc binding induce transition of tomato abscisic acid stress ripening 1, a water stress- and salt stress-regulated plant-specific protein, from unfolded to folded state[J]. Plant Physiology, 2007, 143(2):617-628.
PMID |
| [27] |
Cakir B, Agasse A, Gaillard C, et al. A grape ASR protein involved in sugar and abscisic acid signaling[J]. The Plant Cell, 2003, 15(9):2165-2180.
DOI URL |
| [28] |
Kalifa Y A, Gilad Z, Konrad M, et al. The water- and salt-stress-regulated Asr1(abscisic acid stress ripening) gene encodes a zinc-dependent DNA-binding protein[J]. The Biochemical journal, 2004, 381(Pt2):373-378.
DOI URL |
| [29] |
Nie H S, Wang Y L, Wei C C, et al. Embryogenic Calli Induction and Salt Stress Response Revealed by RNA-Seq in Diploid Wild Species Gossypium sturtianum and Gossypium Raimondi[J]. Frontiers in Plant Science, 2021, 12 : 715041-715041.
DOI URL |
| [30] | Weng Q Y, Zhao Y M, Zhao Y N, et al. Identification of Salt Stress-Responsive Proteins in Maize (Zea may) Seedlings Using iTRAQ-Based Proteomic Technique[J]. Iranian Journal of Biotechnology, 2021, 19(1) : e2512-e2512. |
| [31] |
陈娜, 胡冬青, 潘丽娟, 等. 花生中胁迫相关基因AhDHNI的克隆及非生物胁迫下表达分析[J]. 核农学报, 2014, 28(12):2159-2166.
DOI |
| CHEN Na, HU Dongqin, PAN Lijuan, et al. Cloning of a Dehydrin Gene AhDHN1 and Its Expression Analysis during Abiotic Stresses in Peanut[J]. Journal of Nuclear Agricultural Sciences, 2014, 28(12):2159-2166. | |
| [32] |
Chourey K, Ramani S, Apte S K. Accumulation of LEA proteins in salt (NaCl) stressed young seedlings of rice (Oryza sativa L.) cultivar Bura Rata and their degradation during recovery from salinity stress[J]. Journal of Plant Physiology, 2003, 160(10):1165-1174.
PMID |
| [33] |
Jia F J, Qi S D, Li H, et al. Overexpression of late embryogenesis abundant 14 enhances Arabidopsis salt stress tolerance[J]. Biochemical and Biophysical Research Communications, 2014, 454(4):505-511.
DOI URL |
| [34] |
Wang M Z, Li P, Li C, et al. SiLEA14, a novel atypical LEA protein, confers abiotic stress resistance in foxtail millet[J]. BMC Plant Biology, 2014, 14(1):290.
DOI URL |
| [35] |
Park S C, Kim Y H, Jeong J C, et al. Sweetpotato late embryogenesis abundant 14 (IbLEA14) gene influences lignifications and increases osmotic and salt stress-tolerance of transgenic calli[J]. Planta, 2011, 233(3):621-634.
DOI URL |
| [36] | Yu H T, Wang T. Proteomic dissection of endosperm starch granule associated proteins reveals a network coordinating starch biosynthesis and amino acid metabolism and glycolysis in rice endosperms[J]. Frontiers in Plant Science, 2016, 7:707. |
| [37] |
Wang N B, Zhao J, He X Y, et al. Comparative proteomic analysis of drought tolerance in the two contrasting Tibetan wild genotypes and cultivated genotype[J]. BMC Genomics, 2015, 16(1):432.
DOI URL |
| [38] |
Maeda H, Dudareva N. The shikimate pathway and aromatic amino acid biosynthesis in plants[J]. Annual Review of Plant Biology, 2012, 63(1):73-105.
DOI URL |
| [39] |
Jander G, Joshi V. Recent progress in deciphering the biosynthesis of aspartate derived amino acids in plants[J]. Molecular Plant, 2010, 3(1):54-65.
DOI PMID |
| [40] |
Rothman S. How is the balance between protein synthesis and degradation achieved?[J]. Theoretical Biology and Medical Modelling, 2010, 7(1):25.
DOI URL |
| [41] |
Christiane R, Stephen P, Steffen R. Singlet oxygen signaling links photosynthesis to translation and plant growth[J]. Trends in Plant Science, 2010, 15(9):499-506.
DOI PMID |
| [42] |
Houda M-D, Houhamed D, Laure J, et al. An Arabidopsis mutant disrupted in ASN2 encoding asparagine synthetase 2 exhibits low salt stress tolerance[J]. Plant Physiology and Biochemistry, 2011, 49(6):623-628.
DOI URL |
| [43] |
Wang H B, Liu D C, Sun J Z, et al. Asparagine synthetase gene TaASN1 from wheat is up-regulated by salt stress, osmotic stress and ABA[J]. Journal of Plant Physiology, 2005, 162(1):81-89.
DOI URL |
| [44] | Conroy C, Ching J, Gao Y, et al. Knockout of AtMKK1 enhances salt tolerance and modifies metabolic activities in Arabidopsis[J]. Plant signaling & behavior, 2013, 8(5):e24206. |
| [45] |
Bouche N, Fromm H. GABA in plants: Just a metabolite?[J]. Trends in Plant Science, 2004, 9(3):110-115.
DOI URL |
| [46] |
Michaeli S, Fromm H. Closing the loop on the GABA shunt in plants: are GABA metabolism and signaling entwined?[J]. Frontiers in Plant Science, 2015, 6:419.
DOI PMID |
| [47] | 周翔, 吴晓岚, 李云, 等. 盐胁迫下玉米幼苗ABA和GABA的积累及其相互关系[J]. 应用与环境生物学报, 2005, 11(4):412-415. |
| ZHOU Xiang, WU Xiaolan, LI Yun, et al. Accumulations and Correlations of ABA And GABA in Maize Seedling under Salt Stress[J]. Chinese Journal of Applied & Environmental Biology, 2005, 11(4):412-415. | |
| [48] |
Achard P, Cheng H, Liesbeth D G, et al. Integration of plant responses to environmentally activated phytohormonal signals[J]. Science, 2006, 311(5757):91-94.
DOI URL |
| [49] |
Li C Z, Jiao J, Wang G X. The important roles of reactive oxygen species in the relationship between ethylene and polyamines in leaves of spring wheat seedlings under root osmotic stress[J]. Plant Science, 2003, 166(2):303-315.
DOI URL |
| [50] |
Gil-Amado J A, Gomez-Jimenez M C. Regulation of polyamine metabolism and biosynthetic gene expression during olive mature-fruit abscission[J]. Planta, 2012, 235(6):1221-1237.
DOI PMID |
| [51] |
Farooq M, Wahid A N, Kobayashi D. Plant drought stress: effects, mechanisms and management[J]. Agronomy for Sustainable Development, 2009, 29(1):185-212.
DOI URL |
| [52] |
Zenda T, Liu S T, Wang X, et al. Comparative proteomic and physiological analyses of two divergent maize inbred lines provide more insights into drought-stress tolerance mechanisms[J]. International Journal of Molecular Sciences, 2018, 19(10):3225.
DOI URL |
| [53] |
Rasoulnia A, Bihamta M R, Peyghambari S A, et al. Proteomic response of barley leaves to salinity[J]. Molecular Biology Reports, 2011, 38(8):5055-5063.
DOI URL |
| [54] |
Klaus K, Heribert H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction[J]. Annual Review of Plant Biology, 2004, 55(1):373-399.
DOI URL |
| [55] |
Westermann S, Weber K. Post-translational modifications regulate microtubule function.[J]. Nature Reviews Molecular Cell Biology, 2003, 4(12): 938.
PMID |
| [56] |
Gaertig J, Verhey F. The tubulin code[J]. Cell Cycle, 2007, 6 (17):2152-2160.
DOI URL |
| [1] | 徐毛毛, 高杰, 李君明, 李鑫, 刘磊, 潘峰. 20个番茄商业品种群体的多样性分析[J]. 新疆农业科学, 2024, 61(9): 2191-2196. |
| [2] | 田海燕, 张占琴, 颉建辉, 王建江, 杨相昆. 加工番茄果实番茄红素与主要品质性状的关系[J]. 新疆农业科学, 2024, 61(9): 2197-2202. |
| [3] | 田超, 李玉姗, 马越, 宋羽. 不同浓度苦豆子浸提液对连作番茄生长及土壤肥力的影响[J]. 新疆农业科学, 2024, 61(9): 2203-2210. |
| [4] | 陈芳, 李字辉, 孙孝贵, 张庭军. 不同剂量的微生物菌剂对加工番茄产量及品质的影响[J]. 新疆农业科学, 2024, 61(9): 2285-2289. |
| [5] | 董志多, 徐菲, 付秋萍, 黄建, 祁通, 孟阿静, 付彦博, 开赛尔·库尔班. 不同类型盐碱胁迫对棉花种子萌发的影响[J]. 新疆农业科学, 2024, 61(8): 1831-1844. |
| [6] | 奚瑞, 陈怡佳, 李宁, 余庆辉, 王强, 秦勇. 外源2, 4-表芸苔素内酯对盐胁迫下不同盐敏感型番茄种子萌发的影响[J]. 新疆农业科学, 2024, 61(8): 1983-1992. |
| [7] | 张彩虹, 王国强, 姜鲁艳, 刘涛, 德贤明. 低能耗组装式深冬生产型日光温室环境因子变化及番茄性状分析[J]. 新疆农业科学, 2024, 61(8): 2043-2053. |
| [8] | 张福林, 李宁, 刘宇翔, 陈怡佳, 余庆辉, 闫会转. 外源2,4-表油菜素内酯及褪黑素对樱桃番茄果实品质和果皮形态结构的影响[J]. 新疆农业科学, 2024, 61(7): 1738-1747. |
| [9] | 阮向阳, 蒲敏, 肖乐乐, 罗林毅, 陈瑞杰, 李然, 陈国永, 冶军. 镁肥施用策略对加工番茄产量和品质的影响[J]. 新疆农业科学, 2024, 61(4): 916-925. |
| [10] | 欧源, 罗莎莎, 王如月, 孙雅丽, 虎海防. 盐胁迫对美国黑核桃幼苗生长和生理特性的影响[J]. 新疆农业科学, 2024, 61(2): 393-401. |
| [11] | 李春雨, 谭占明, 程云霞, 高源, 马全会, 李志国, 马兴. 水肥耦合对沙培番茄叶绿素含量以及光合特性日变化的影响[J]. 新疆农业科学, 2024, 61(12): 3006-3013. |
| [12] | 李亚莉, 哈丽哈什·依巴提, 唐亚莉, 段婧婧, 李青军. 氮磷减施与钾协同共效对加工番茄产量和养分吸收的影响[J]. 新疆农业科学, 2024, 61(12): 3014-3019. |
| [13] | 刘会芳, 王强, 韩宏伟, 庄红梅, 王浩, 常亚南. 盐、碱及复合盐碱胁迫对番茄幼苗光合特性及抗氧化酶活性的影响[J]. 新疆农业科学, 2024, 61(11): 2658-2666. |
| [14] | 赵文轩, 程云霞, 谭占明, 李春雨, 束胜, 阿依买木·沙吾提, 杨历雨, 苗献军. 基于主成分分析比较不同加工番茄品种叶绿素的荧光参数及光合特性[J]. 新疆农业科学, 2024, 61(11): 2667-2675. |
| [15] | 李春雨, 谭占明, 程云霞, 束胜, 马全会, 何淼, 段轶帆, 吴慧. 不同加工番茄品种的农艺性状比较分析[J]. 新疆农业科学, 2024, 61(11): 2676-2683. |
| 阅读次数 | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
全文 50
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
摘要 155
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||