

新疆农业科学 ›› 2024, Vol. 61 ›› Issue (4): 937-944.DOI: 10.6048/j.issn.1001-4330.2024.04.018
• 植物保护·微生物·农业装备工程与机械化 • 上一篇 下一篇
吴基楠1,2( ), 董婉莹2,3, 刘同先1, 王冰2(
), 董婉莹2,3, 刘同先1, 王冰2( ), 王桂荣2(
), 王桂荣2( )
)
收稿日期:2023-08-11
出版日期:2024-04-20
发布日期:2024-05-31
通信作者:
王桂荣(1972-),男,安徽宿松人,研究员,研究方向为化学生态学,(E-mail)wangguirong@caas.cn;作者简介:吴基楠(1997-),男,福建三明人,硕士研究生,研究方向为化学生态学,(E-mail)wujinan107@163.com
基金资助:
WU Jinan1,2( ), DONG Wanying2,3, LIU Tongxian1, WANG Bing2(
), DONG Wanying2,3, LIU Tongxian1, WANG Bing2( ), WANG Guirong2(
), WANG Guirong2( )
)
Received:2023-08-11
Published:2024-04-20
Online:2024-05-31
Correspondence author:
WANG Guirong (1972-), male, from Susong, Anhui,researcher, research direction: chemical ecology, (E-mail)wangguirong@caas.cn;Supported by:摘要:
【目的】分析大灰优蚜蝇Eupeodes corollae雌成虫胸足的感器类型、特征及分布,为天敌昆虫食蚜蝇产卵选择机制奠定形态学基础。【方法】利用扫描电镜技术观察其足上感器的超微结构。【结果】大灰优蚜蝇雌成虫胸足由基节、转节、腿节、胫节、跗节和前跗节6个部分组成,其上分布5种类型的感器,分别为锥形感器(3种亚型I~III)、毛形感器(长毛形感器2种亚型I~II;短毛形感器2种亚型I~II)、刺形感器(2种亚型I~II)、Böhm氏鬃毛和微毛感器。锥形感器仅分布于足跗节,其中锥形感器I亚型顶端具孔,在化学感受过程中发挥重要的作用;毛形感器和刺形感器在雌成虫胸足腿节、胫节和跗节均有分布,这2种类型感器的毛干表面有纵脊,无壁孔,基部具可活动的臼状窝,是典型的机械感器;Böhm 氏鬃毛和微毛感器数量较多,散布在各类感器之间。【结论】在大灰优蚜蝇雌成虫胸足上鉴定了5种类型的感器,足跗节上的感器类型和数量最多,是足上重要的化学感受区域。
中图分类号:
吴基楠, 董婉莹, 刘同先, 王冰, 王桂荣. 大灰优蚜蝇雌成虫足感器扫描电镜分析[J]. 新疆农业科学, 2024, 61(4): 937-944.
WU Jinan, DONG Wanying, LIU Tongxian, WANG Bing, WANG Guirong. Ultrastructural observations of leg sensilla in Eupeodes corollae using scanning electron microscopy[J]. Xinjiang Agricultural Sciences, 2024, 61(4): 937-944.
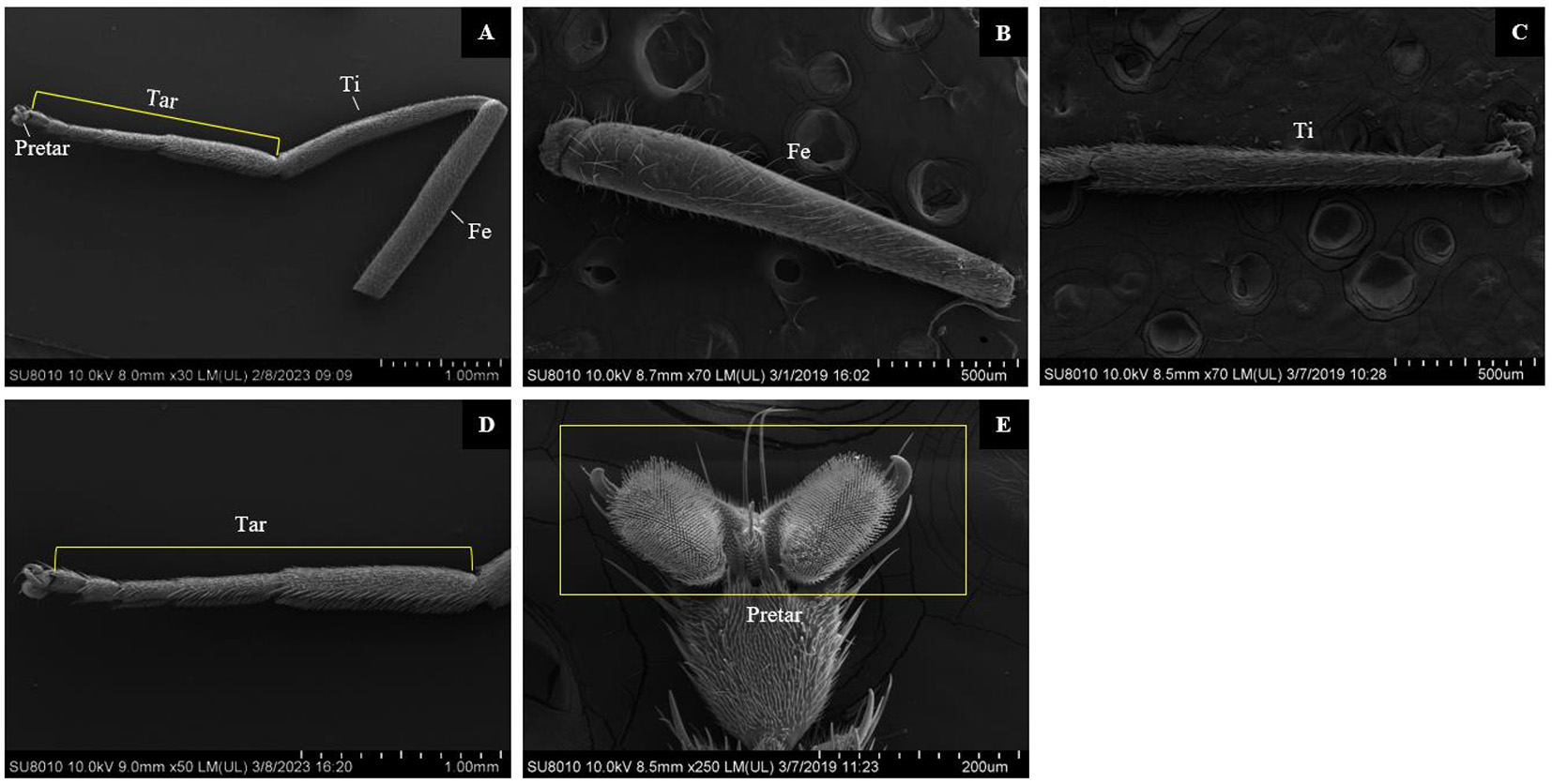
图1 大灰优蚜蝇雌虫前足扫描电镜图 注:A. 大灰优蚜蝇足部分整体图,由腿节、胫节、跗节和前跗节构成;B. 腿节整体图;C. 胫节整体图;D. 跗节整体图;E. 前跗节整体图(黄色矩形边框);腿节Femur(Fe);胫节Tibia(Ti);跗节Tarsus(Tar);前跗节Pretarsus(Pretar)
Fig.1 Scanning electron micrographs of foreleg in female adults of Eupeodes corollae Note: A. General view of the part of leg from Eupeodes corollae, showing the femur, the tibia, the tarsus, the pretarsus; B. General view of the femur; C. General view of the tibia; D. General view of the tarsus; E. General view of the pretarsus (yellow rectangular border); Femur (Fe); Tibia (Ti); Tarsus (Tar); Pretarsus (Pretar)

图2 大灰优蚜蝇雌虫前足腿节和胫节扫描电镜图 注:A. 位于腿节的毛形感器;B. 长毛形感器 I型long sensilla trichodea I(LST I)和短毛形感器 I型short sensilla trichodea I(SST I);C. Böhm 氏鬃毛(BB),毛形感器(ST)基部的表皮窝;D. 位于胫节的刺形感器(SC)和Böhm 氏鬃毛(BB)
Fig.2 Scanning electron micrographs of femur and tibia in foreleg of female adults of E. corollae Note: A. Sensilla trichodea in the femur; B. Long sensilla trichodea I (LST I) and short sensilla trichodea I (SST I); C. Böhm bristles (BB), epidermal fossa at the base of sensilla trichodea (ST); D. Sensilla chaetica (SC) and böhm bristles (BB) at the tibia

图3 大灰优蚜蝇雌虫前足跗节扫描电镜图 注:A. 位于跗节背面的短刺形感器short sensilla chaetica(SSC)和长刺形感器long sensilla chaetica(LSC);B. 位于跗节两侧的短刺形感器(SSC)和长刺形感器(LSC);C. 位于跗节的短刺形感器(SSC)和Böhm 氏鬃毛(BB);D. 微毛感器(Mt);E. 位于跗节腹面的长毛形感器 Ⅱ型long sensilla trichodea Ⅱ(LST Ⅱ)和短毛形感器 Ⅱ型short sensilla trichodea Ⅱ(SST Ⅱ);F. 跗节腹面的锥形感器(SB)SB I,SB II和SB III亚型;G. 锥形感器 I型(SB I);H. 锥形感器 II型(SB II);I. 锥形感器 III型(SB III)
Fig.3 Scanning electron micrographs of tarsus in foreleg of female adults of E. corollae Note: A. Short sensilla chaetica (SSC) and long sensilla chaetica (LSC) at the back of the tarsus; B. Short sensilla chaetica (SSC) and long sensilla chaetica (LSC) at the both sides of the tarsus; C. Short sensilla chaetica (SSC) and böhm bristles (BB) at the tarsus; D. Microtrichiae (Mt); E. Long sensilla trichodea Ⅱ (LST Ⅱ) and short sensilla trichodea Ⅱ (SST Ⅱ) at the ventral sides of the tarsus; E. SB I, SB II and SB III at the ventral sides of the tarsus; G. SB I; H. SB II; I. SB III
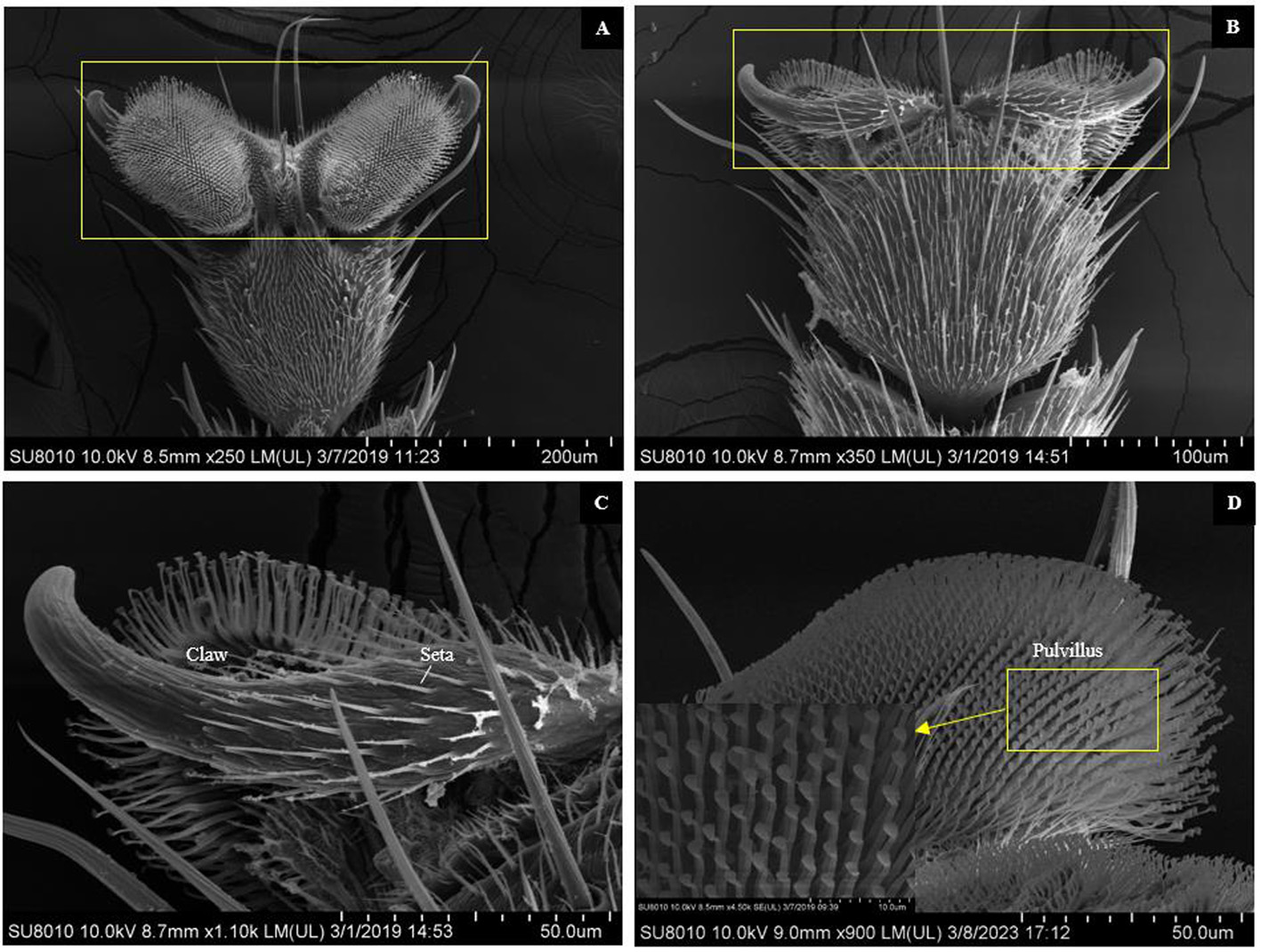
图4 大灰优蚜蝇雌虫前足前跗节扫描电镜图 注:A. 黄色框为前跗节的腹面图;B. 黄色框为前跗节的背面图;C. 位于前跗节背面的爪(claw)和刚毛(seta);D. 位于前跗节腹面的爪垫(puvillus)
Fig.4 Scanning electron micrographs of pretarsus in foreleg of female adults of E. corollae Note: A. General view of ventral view of the pretarsus (yellow rectangular border); B. General view of back view of the pretarsus (yellow rectangular border); C. Claw and seta at the back side of the pretarsus; D. Puvillus at the ventral side of the pretarsus
| [1] |
Haverkamp A, Hansson B S, Knaden M. Combinatorial codes and labeled lines: how insects use olfactory cues to find and judge food, mates, and oviposition sites in complex environments[J]. Frontiers in Physiology, 2018, 9: 49.
DOI PMID |
| [2] |
Renou M, Anton S. Insect olfactory communication in a complex and changing world[J]. Current Opinion in Insect Science, 2020, 42: 1-7.
DOI PMID |
| [3] |
Montell C. Drosophila sensory receptors-a set of molecular Swiss Army Knives[J]. Genetics, 2021, 217(1): 1-34.
DOI PMID |
| [4] |
Sokolinskaya E L, Kolesov D V, Lukyanov K A, et al. Molecular principles of insect chemoreception[J]. Acta Naturae, 2020, 12(3): 81-91.
DOI PMID |
| [5] | Auer T O, Khallaf M A, Silbering A F, et al. Olfactory receptor and circuit evolution promote host specialization[J]. Nature, 2020, 579(7799): 402-408. |
| [6] | Amin H, Lin A C. Neuronal mechanisms underlying innate and learned olfactory processing in Drosophila[J]. Current Opinion in Insect Science, 2019, 36: 9-17. |
| [7] | Anholt R R H. Chemosensation and evolution of Drosophila host plant selection[J]. Science, 2020, 23(1): 100799. |
| [8] |
Pannunzi M, Nowotny T. Odor stimuli: not just chemical identity[J]. Frontiers in Physiology, 2019, 10: 1428.
DOI PMID |
| [9] |
Anton S, Rössler W. Plasticity and modulation of olfactory circuits in insects[J]. Cell and Tissue Research, 2021, 383(1): 149-164.
DOI PMID |
| [10] | Chin S G, Maguire S E, Huoviala P, et al. Olfactory neurons and brain centers directing oviposition decisions in Drosophila[J]. Cell Reports, 2018, 24(6): 1667-1678. |
| [11] | Patel M, Rangan A. Olfactory encoding within the insect antennal lobe: the emergence and role of higher order temporal correlations in the dynamics of antennal lobe spiking activity[J]. Journal of Theoretical Biology, 2021, 522: 110700. |
| [12] |
Schultzhaus J N, Saleem S, Iftikhar H, et al. The role of the Drosophila lateral horn in olfactory information processing and behavioral response[J]. Journal of Insect Physiology, 2017, 98: 29-37.
DOI PMID |
| [13] | Gao H H, Lai S G, Zhai Y F, et al. Comparison of the antennal sensilla and compound eye sensilla in four Drosophila (Diptera: Drosophilidae) species[J]. Florida Entomologist, 2020, 102(4): 747. |
| [14] | Hore G, Maity A, Naskar A, et al. Scanning electron microscopic studies on antenna of Hemipyrellia ligurriens (Wiedemann, 1830) (Diptera: Calliphoridae)—a blow fly species of forensic importance[J]. Acta Tropica, 2017, 172: 20-28. |
| [15] | Jia H R, Sun Y F, Luo S P, et al. Characterization of antennal chemosensilla and associated odorant binding as well as chemosensory proteins in the Eupeodes corollae (Diptera: Syrphidae)[J]. Journal of Insect Physiology, 2019, 113: 49-58. |
| [16] | Liu F, Wang Q, Xu P, et al. A dual-target molecular mechanism of pyrethrum repellency against mosquitoes[J]. Nature Communications, 2021, 12(1): 2553. |
| [17] |
Smallegange R C, Kelling F J, Den Otter C J. Types and numbers of sensilla on antennae and maxillary palps of small and large houseflies, Musca domestica (Diptera, Muscidae)[J]. Microscopy Research and Technique, 2008, 71(12): 880-886.
DOI PMID |
| [18] | Jiao Y C, Moon S J, Wang X Y, et al. Gr64f is required in combination with other gustatory receptors for sugar detection in Drosophila[J]. Current Biology: CB, 2008, 18(22): 1797-1801. |
| [19] |
Lee Y, Kim S H, Montell C. Avoiding DEET through insect gustatory receptors[J]. Neuron, 2010, 67(4): 555-561.
DOI PMID |
| [20] | Miyamoto T, Wright G, Amrein H. Nutrient sensors[J]. Current Biology, 2013, 23(9): R369-R373. |
| [21] | Moon S J, Lee Y, Jiao Y C, et al. A Drosophila gustatory receptor essential for aversive taste and inhibiting male-to-male courtship[J]. Current Biology: CB, 2009, 19(19): 1623-1627. |
| [22] | Sollai G, Tomassini Barbarossa I, Solari P, et al. Taste discriminating capability to different bitter compounds by the larval styloconic sensilla in the insect herbivore Papilio hospiton (Géné)[J]. Journal of Insect Physiology, 2015, 74: 45-55. |
| [23] | Zhang Y F, Huang L Q, Ge F, et al. Tarsal taste neurons of Helicoverpa assulta (Guenée) respond to sugars and amino acids, suggesting a role in feeding and oviposition[J]. Journal of Insect Physiology, 2011, 57(10): 1332-1340. |
| [24] | Zhang Y F, van Loon J J A, Wang C Z. Tarsal taste neuron activity and proboscis extension reflex in response to sugars and amino acids in Helicoverpa armigera (Hubner)[J]. The Journal of Experimental Biology, 2010, 213(Pt 16): 2889-2895. |
| [25] | Ling F, Dahanukar A, Weiss L A, et al. The molecular and cellular basis of taste coding in the legs ofDrosophila[J]. The Journal of Neuroscience, 2014, 34(21): 7148-7164. |
| [26] | Klecka J, Hadrava J, Biella P, et al. Flower visitation by hoverflies (Diptera: Syrphidae) in a temperate plant-pollinator network[J]. PeerJ, 2018, 6: e6025. |
| [27] |
Rader R, Bartomeus I, Garibaldi L A, et al. Non-bee insects are important contributors to global crop pollination[J]. Proceedings of the National Academy of Sciences of the United States of America, 2016, 113(1): 146-151.
DOI PMID |
| [28] | Dong W Y, Wang B, Wang G R. Morphological and ultrastructural characterization of antennal sensilla and the detection of floral scent volatiles in Eupeodes corollae (Diptera: Syrphidae)[J]. Frontiers in Neuroanatomy, 2021, 15: 791900. |
| [29] | Wang B, Dong W Y, Li H M, et al. Molecular basis of (E)-β-farnesene-mediated aphid location in the predator Eupeodes corollae[J]. Current Biology, 2022, 32(5): 951-962.e7. |
| [30] | Hood Henderson D E, Wellington W G. Antennal sensilla of some aphidophagous Syrphidae (Diptera): fine structure and electroantennogramme study[J]. Canadian Journal of Zoology, 1982, 60(12): 3172-3186. |
| [31] |
Bisotto-de-Oliveira R, Redaelli L R, Sant’ana J. Morphometry and distribution of sensilla on the antennae of Anastrepha fraterculus (Wiedemann) (Diptera: Tephritidae)[J]. Neotropical Entomology, 2011, 40(2): 212-216.
DOI PMID |
| [32] |
Pezzi M, Whitmore D, Chicca M, et al. Ultrastructural morphology of the antenna and maxillary palp of Sarcophaga tibialis (Diptera: Sarcophagidae)[J]. Journal of Medical Entomology, 2016, 53(4): 807-814.
PMID |
| [33] | Dey S, Hooroo R N K, Wankhar D. Scanning electron microscopic studies of the external morphology of sensilla on the legs of a butterfly, Graphium sarpedon (Lepidoptera—Papillionidae)[J]. Micron, 1995, 26(5): 367-376. |
| [34] | Takai H, Asaoka K, Ishizuna F, et al. Morphological and electrophysiological differences in tarsal chemosensilla between the wild silkmoth Bombyx mandarina and the domesticated species Bombyx mori[J]. Arthropod Structure & Development, 2018, 47(3): 238-247. |
| [35] | Ni L N, Bronk P, Chang E C, et al. A gustatory receptor paralogue controls rapid warmth avoidance in Drosophila[J]. Nature, 2013, 500(7464): 580-584. |
| [36] | Schneider D. Insect antennae[J]. Annual Review of Entomology, 1964, 9: 103-122. |
| [37] | Zhang G N, Hu F, Dou W, et al. Morphology and distribution of sensilla on tarsi and ovipositors of six fruit flies (Diptera: Tephritidae)[J]. Annals of the Entomological Society of America, 2012, 105(2): 319-327. |
| [38] |
Silva D S, Barp E A, Kucharski L C R, et al. Sensing the plant surface prior to feeding and oviposition: differences in external ultrastructure and function among tarsi of Heliconius erato[J]. Neotropical Entomology, 2018, 47(1): 85-95.
DOI PMID |
| [39] |
McIver S, Siemicki R. Fine structure of tarsal sensilla of Aedes aegypti (L.) (Diptera: Culicidae)[J]. Journal of Morphology, 1978, 155(2): 137-155.
DOI PMID |
| [40] | 王争艳, 莫建初. 雌家蝇产卵器和足上感受器的形态学研究[J]. 中国媒介生物学及控制杂志, 2010, 21(2): 115-116, 120. |
| WANG Zhengyan, MO Jianchu. Morphological study of the sensilla on ovipositors and legs of female houseflies[J]. Chinese Journal of Vector Biology and Control, 2010, 21(2): 115-116, 120. | |
| [41] | Dweck H K M, Gadallah N S. Description of the antennal sensilla of Habrobracon hebetor[J]. BioControl, 2008, 53(6): 841-856. |
| [42] |
Stocker R F. The organization of the chemosensory system in Drosophila melanogaster: a review[J]. Cell and Tissue Research, 1994, 275(1): 3-26.
PMID |
| [43] | 杨科, 王琛柱. 昆虫味觉研究进展及相关原理在害虫防治中的应用[J]. 应用昆虫学报, 2023, 60(2): 486-498. |
| YANG Ke, WANG Chenzhu. Progress in insect gustatory research and the application of related principles to pest control[J]. Chinese Journal of Applied Entomology, 2023, 60(2): 486-498. | |
| [44] |
Chen Y, Amrein H. Ionotropic receptors mediate Drosophila oviposition preference through sour gustatory receptor neurons[J]. Current Biology: CB, 2017, 27(18): 2741-2750.e4.
DOI PMID |
| [45] | Zhang L W, Sun H W, Grosse-Wilde E, et al. Cross-generation pheromonal communication drives Drosophila oviposition site choice[J]. Current Biology: CB, 2023, 33(10): 2095-2103.e3. |
| [46] | Zhang L W, Yu J, Guo X, et al. Parallel mechanosensory pathways direct oviposition decision-making in Drosophila[J]. Current Biology: CB, 2020, 30(16): 3075-3088. |
| [1] | 赵晓梅;吴玉鹏;陈维维;叶凯. 库尔勒香梨采后萼端黑斑病发病果实果肉细胞超微结构和生理活性的变化[J]. , 2016, 53(9): 1640-1646. |
| [2] | 李银;张辉;骆建敏;袁海英;薛珊珊;张茜. 蟠桃果实发育成熟过程中果肉细胞超微结构的变化[J]. , 2011, 48(6): 1006-1010. |
| [3] | 王岩;马纪;刘小宁;骆建敏. 苏氏宽漠甲Sternoplax souvorowiana卵的形态和卵壳超微结构[J]. , 2010, 47(9): 1703-1708. |
| [4] | 崔卫东;龙宣杞;侯新强;杨蓉;补娟;罗明. 黄萎病原菌胁迫对丛枝菌根化棉花幼苗根部防御性酶及超微结构的影响[J]. , 2009, 46(6): 1235-1244. |
| [5] | 克热木·伊力;侯江涛. 盐胁迫对甜土植物营养器官显微及超微结构的研究进展[J]. , 2006, 43(3): 234-236. |
| 阅读次数 | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
全文 39
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
摘要 213
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||